Organic chemistry is the study of compounds that contain carbon. These compounds form the basis…

The Ultimate Guide to Teaching Oxidation Numbers in Your Online Chemistry Class
Oxidation numbers, also known as oxidation states, help determine what is being oxidized and reduced in a redox reaction. It’s one of those chemistry topics that seem simple on paper as it’s just assigning a number to an element, but the rules and exceptions can quickly become confusing.
This article is here to help you teach oxidation numbers with clear rules, strong patterns, and a few memory tricks. Help your students truly understand these numbers, rather than just memorizing them.
Introducing redox reactions and oxidation numbers
A redox reaction is a chemical reaction that involves the transfer of electrons.
- Oxidation: loss of electrons (oxidation number increases)
- Reduction: gain of electrons (oxidation number decreases)
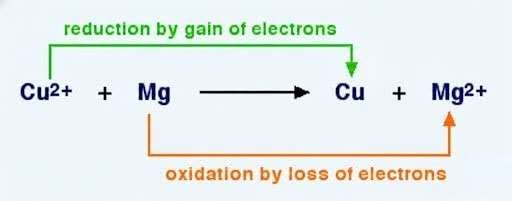
If one substance is oxidized, another must be reduced. This always happens together. A classic trick to remember which process is loss and which process is gain is “LEO (lose elections oxidation) the lion says GER (gain electrons reduction)”.
An oxidation number is a number assigned to an atom that represents how many electrons it has effectively lost or gained compared to its neutral state. They don’t always represent real charges, but they help us recognize redox reactions and balance redox equations.
Oxidation number rules
Students don’t need a ton of rules to get familiar with oxidation numbers. They just need a small, reliable hierarchy. Teach these rules in order and emphasize that earlier rules take priority over later ones.
1) Free elements = 0
Any element by itself, in its standard state, has an oxidation number of 0.
Examples:
- Na(s) → 0
O2 → 0
- Cl2 → 0
Remember, HOFBrINCl (pronounced “hoff brincle”) for the seven diatomic elements that always exist in two’s in their standard states: H2, O2, F2, Br2, I2, N2, Cl2.
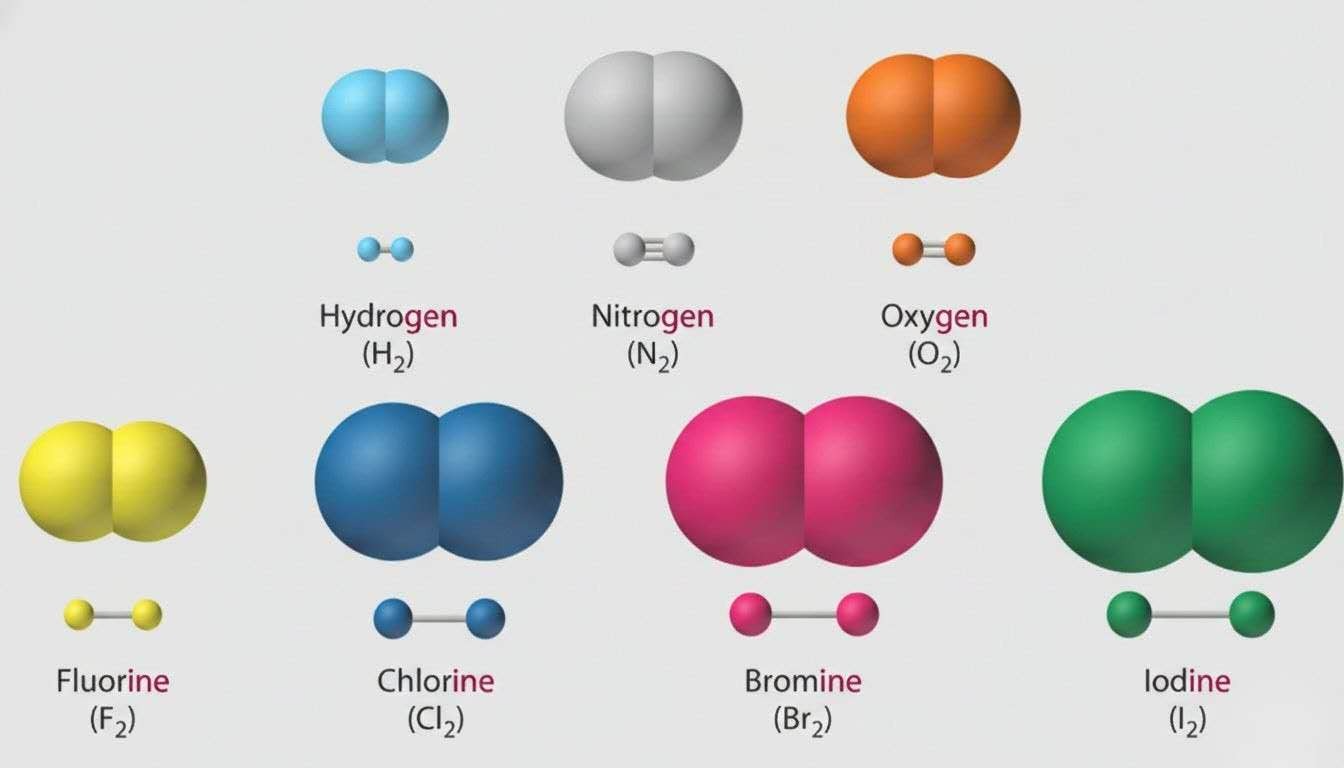
Memory tip: If it’s alone, it’s zero.
2) Monoatomic ions = charge
For single-atom ions, the oxidation number equals the ion’s charge.
Examples:
- Na+ → +1
- Mg2+ → +2
- Cl- → -1
Memory tip: Ion charge = oxidation charge.
3) Group 1 metals are always +1
Lithium, sodium, potassium, and all the other alkali metals have one valence electron, so to be stable, they need to give up that one electron.
Examples:
- Na in NaCl → +1
- K in K2SO4 → +1
Memory tip: Group 1 gives up 1.
4) Group 2 metals are always +2
Magnesium, calcium, and all other alkaline earth metals have two valence electrons, so to be stable, they need to give up two electrons.
Examples:
- Mg in MgO → +2
- Ca in CaCl2 → +2
Memory tip: Group 2 gives up 2.
5) Fluorine is always -1
No exceptions to this one. Fluorine is the most electronegative element on the periodic table. It has 7 valence electrons and it will do whatever it takes to gain one more electron.
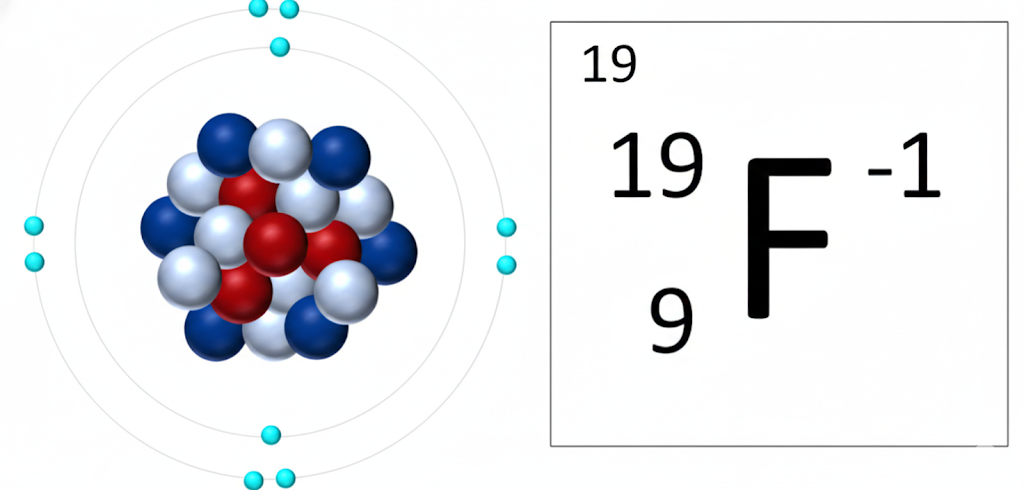
Memory tip: Fluorine is Forever -1.
6) Oxygen is usually -2
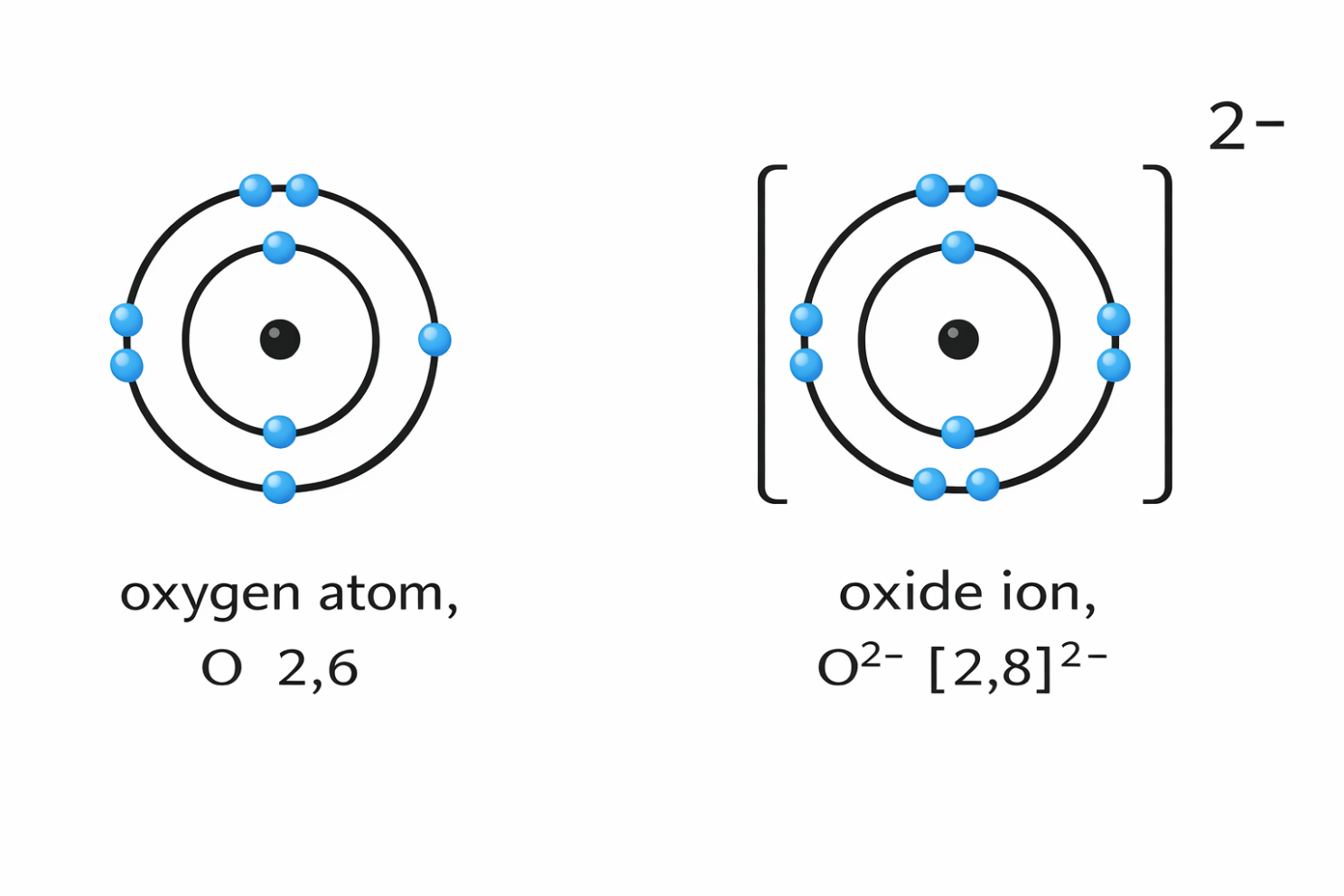
When bonded to most elements, oxygen has an oxidation state of -2 (as it needs to gain two electrons to be stable), but there are a few exceptions which students will have to remember:
- Peroxides (e.g. H2O2) → -1
- OF2 (bonded to fluorine): → +2 (because remember, fluorine is always -1)
Memory tip: Oxygen loves -2…unless it’s in a peroxide or bonded to F.
7) Hydrogen is usually +1
Hydrogen is the first element on the periodic table, so it has just one proton and one electron. In most cases, hydrogen loses its electron and becomes a proton (H⁺). An important exception is in metal hydrides: because metals are more willing to lose electrons, hydrogen instead gains one, forming H⁻. This still satisfies hydrogen, as it now has two electrons filling its first energy shell.
Memory tip: Hydrogen is +1 with non-metals and -1 with metals.
8) The sum of oxidation numbers equals the total charge of the compound
Neutral compounds have a sum of 0.
Examples:
- H2O: Hydrogen is +1 with non-metals, oxygen is -2…2(+1) + (-2) = 0
- NaCl: Sodium is +1, the total charge of the compound is 0…chlorine must be -1
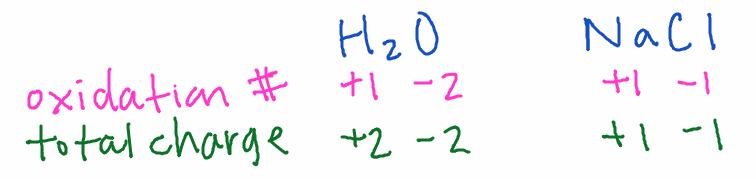
Polyatomic ions have a sum equal to the ionic charge.
Examples:
- SO42-: Oxygen is -2, so the total negative charge is -8. The total polyatomic ion charge is -2, so sulfur must be +6.
- PO43-: Oxygen is -2, so the total negative charge is -8. The total polyatomic ion charge is -3, so phosphorus must be +5
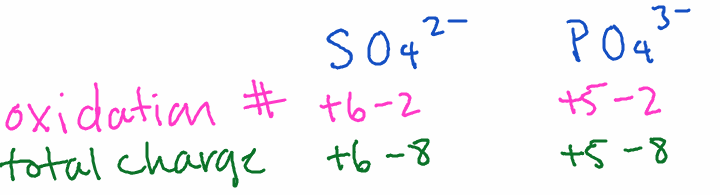
Memory tip: Everything must add up.
Easy patterns for students to notice
Encourage students to look for anchors before calculating anything. These tips can reduce guesswork and build confidence:
- Metals usually have positive oxidation numbers
- Non-metals usually have negative oxidation numbers
- Oxygen and hydrogen appear in most compounds, so use them first
- The unknown oxidation number is often the last one left
Give your students plenty of practice to help them become comfortable assigning oxidation numbers. Once they are more familiar with them, you can move to balancing redox reactions using oxidation numbers, which we will cover in next week’s article, so stay tuned!



