Organic chemistry is often considered one of the most challenging topics in high school and…

The Ultimate Guide to Teaching Functional Groups in Organic Chemistry
Organic chemistry is the study of compounds that contain carbon. These compounds form the basis of life, and their structures and reactions are central to fields like chemistry, biology, medicine, and material science. Since carbon can form four covalent bonds, it’s a versatile element that can create a variety of molecules through bonding with itself or other elements. Organic compounds range from simple gases to complex biomolecules such as DNA and proteins.
A functional group is a specific group of atoms within a molecule that determines how that molecule behaves in chemical reactions. They are the reactive centers of organic molecules and are crucial for predicting properties, naming compounds, and understanding reactivity. When you’re teaching organic chemistry, focusing on functional groups gives students a framework for recognizing patterns across thousands of compounds.
Here are the major functional groups to teach your online chemistry students:
1) Alcohol
- Structure: -OH group attached to a carbon
- Naming: Ends with -ol
- Example: Ethanol (C₂H₅OH)
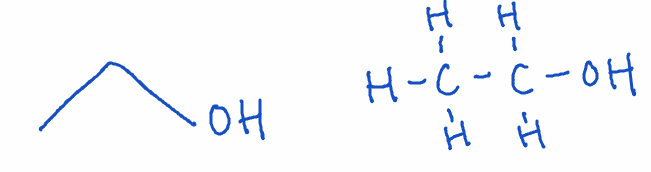
- Key point: Alcohols are polar and can form hydrogen bonds, making them soluble in water. They often appear in everyday substances like beverages and hand sanitizers.
2) Aldehyde
- Structure: Carbonyl group (C=O) at the end of a carbon chain.
- Naming: Ends with -al
- Example: Propanal (C3H6O)
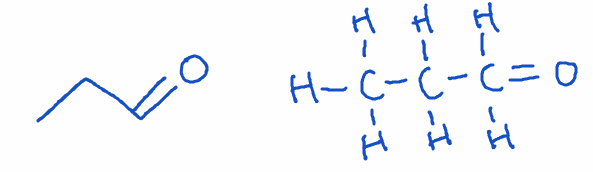
- Key point: Aldehydes are highly reactive and commonly used in preservatives and resins. They can be easily oxidized to carboxylic acids.
3) Ketone
- Structure: Carbonyl group (C=O) within a carbon chain (not at the end).
- Naming: Ends with -one
- Example: Butan-2-one (C4H8O)
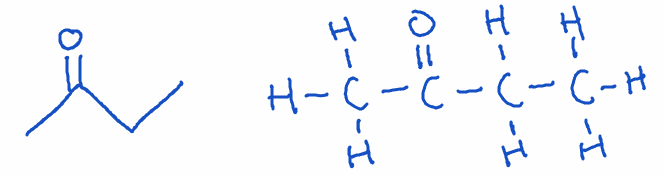
- Key point: Ketones are found in solvents and also occur naturally in the body as ketone bodies during fat metabolism.
4) Ether
- Structure: Oxygen atom bonded to two carbons (R-O-R’)
- Naming: alkoxyalkane
- Example: Methoxyethane (C3H8O)
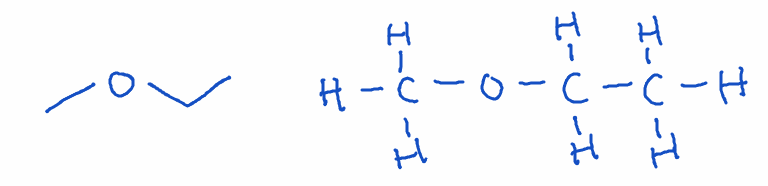
- Key point: Ethers are relatively unreactive and were once widely used as anesthetics. They are also useful as solvents.
5) Carboxylic Acid
- Structure: Carboxyl group (-COOH) at the end of the chain
- Naming: Ends with -oic acid
- Example: Propanoic acid (C3H6O2)
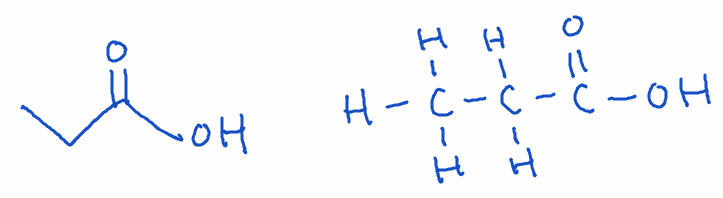
- Key point: Carboxylic acids are acidic due to the ability to donate a proton (H+). They are found in vinegar, citrus fruits, and many other compounds.
6) Ester
- Structure: -COOR group (derived from a carboxylic acid and alcohol)
- Naming: Ends with -oate (two words)
- Example: methyl ethanoate (C2H6O2)
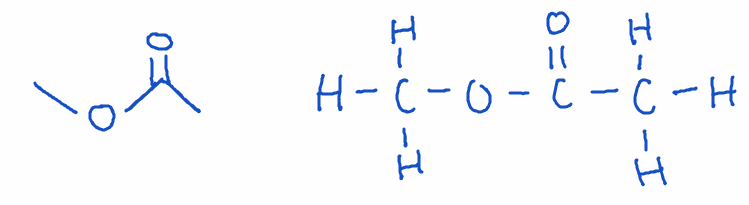
- Key point: Esters have pleasant, fruity smells and are commonly used in perfumes, flavorings, and pharmaceuticals.
7) Amine
- Structure: Nitrogen bonded to one or more carbons (-NH2, -NHR, or -NR2)
- Naming: Ends with -amine
- Example: N-methylethanamine (C3H9N)
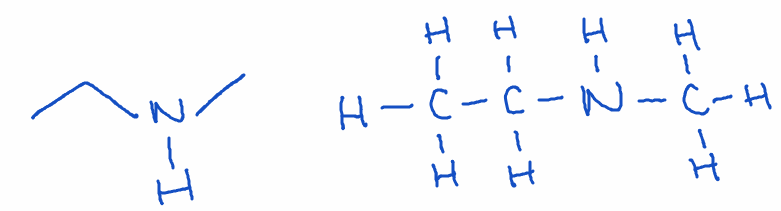
- Key point: Amines are important in biological molecules such as neurotransmitters and amino acids.
8) Amide
- Structure: Carbonyl group (C=O) bonded to nitrogen (-CONH2)
- Naming: Ends with -amide
- Example: N-ethylpropanamide
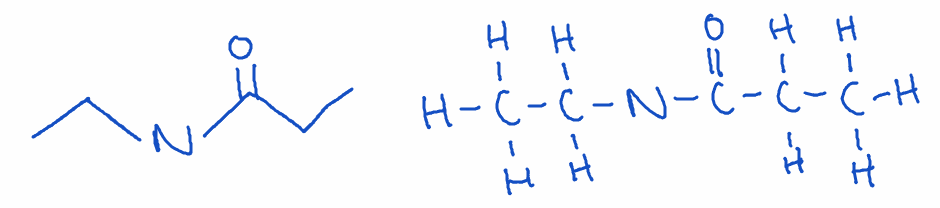
- Key point: Amides are essential in proteins, where peptide bonds link amino acids. They are typically stable and less reactive than esters.
We hope you found this article useful to help you introduce functional groups to your chemistry students! What other chemistry subjects would you like to see covered in our weekly newsletter? Share them in the comments below!



