Last week, we introduced techniques for teaching online chemistry students how to assign oxidation numbers…

How to Teach Balancing Redox Reactions Using the Half-Reaction Method
Now that we’ve covered how to assign oxidation numbers and how to balance redox reactions using the oxidation number method, the next step in your chemistry class is introducing the half-reaction method. This method is more systematic and is especially useful for reactions occurring in acidic or basic solutions.
The half-reaction method makes electron transfer more obvious and works for complex ionic equations. This prepares students for electrochemistry, electrolysis, and advanced redox problems.
The big idea to tell students first is that redox reactions can be split into two parts: oxidation and reduction half-reactions. Each part is balanced separately, and then the two half-reactions are recombined. Now, unlike traditional balancing reactions where only the number of atoms must be balanced, the charges also have to be balanced.
Step-by-Step Process for Balancing Redox Reactions Using the Half-Reaction Method in ACIDIC SOLUTION
While you’re teaching the steps of this method, use an example and actively solve the reaction along with the steps. Students learn best when they see the rules applied immediately.
Let’s take a look at this example together:
Balance the following reaction in acidic solution: Fe2+(aq) + (Cr2O7)2-(aq) → Fe3+(aq) + Cr3+(aq)
Step 1: Split into Oxidation and Reduction Half-Reactions
Tell students that the first step is to separate the reaction into an oxidation half-reaction and a reduction half-reaction. Remember, oxidation involves losing electrons and reduction involves gaining electrons.
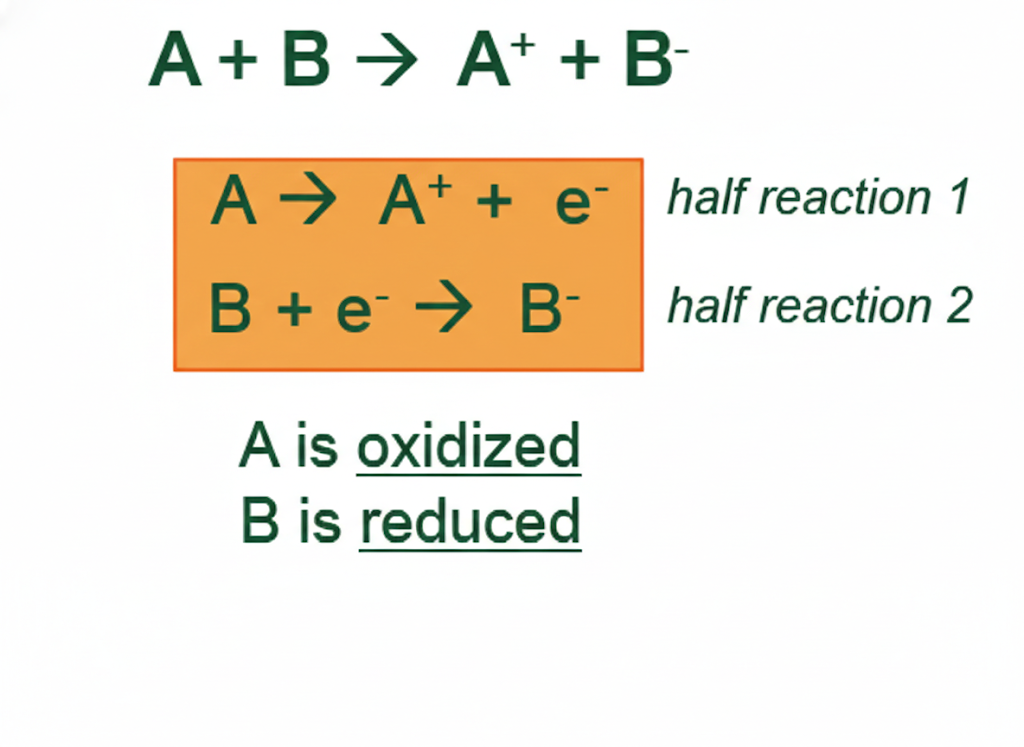
In our example:
Oxidation (Fe is being oxidized): Fe2+(aq) → Fe3+(aq)
Reduction (Cr is being reduced): (Cr2O7)2-(aq) → Cr3+(aq)
This is a great place to quickly review oxidation number changes. An increase in oxidation number means the element in oxidized.
Step 2: Balance All Elements Except Oxygen and Hydrogen
Fe2+(aq) → Fe3+(aq)
(Cr2O7)2-(aq) → 2Cr3+(aq)
Step 3: Balance Oxygen by Adding Water

Fe2+(aq) → Fe3+(aq)
(Cr2O7)2-(aq) → 2Cr3+(aq) + 7H2O(l)
Step 4: Balance Hydrogen by Adding H+
Fe2+(aq) → Fe3+(aq)
(Cr2O7)2-(aq) + 14H+ → 2Cr3+(aq) + 7H2O(l)
Step 5: Balance Charge by Adding Electrons

Fe2+(aq) → Fe3+(aq) + e-
(Cr2O7)2-(aq) + 14H+ + 6e- → 2Cr3+(aq) + 7H2O(l)
Step 6: Make Electron Transfer Equal
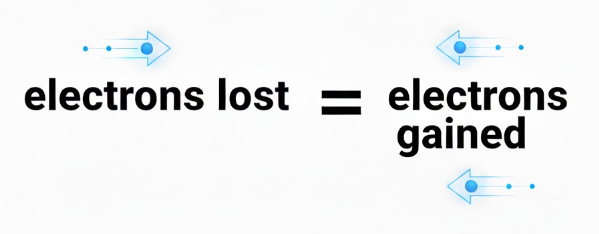
Ensure the total numbers of electrons lost is equal to the total number of electrons gained. Electrons should not be present in the final answer!
The oxidation reaction produces 1 electron and the reduction reaction uses 6 electrons, so multiply the oxidation reaction by 6.
(Fe2+(aq) → Fe3+(aq) + e-) x6
6Fe2+(aq) → 6Fe3+(aq) + 6e-
(Cr2O7)2-(aq) + 14H+ + 6e- → 2Cr3+(aq) + 7H2O(l)
Step 7: Add the Half-Reactions Together
Cancel out the electrons on both sides of the equation.
6Fe2+(aq) + (Cr2O7)2-(aq) + 14H+ + 6e- → 6Fe3+(aq) + 6e- + 2Cr3+(aq) + 7H2O(l)
6Fe2+(aq) + (Cr2O7)2-(aq) + 14H+ → 6Fe3+(aq) + 2Cr3+(aq) + 7H2O(l)
Step 8: Always Do a Final Check

Ensure atoms and charge are balanced and electrons cancel.
6 Fe atoms on each side ✔
2 Cr atoms on each side ✔
7 O atoms on each side ✔
14 H atoms on each side ✔
Total charge of reactant side = 6(+2) + (-2) + 14 = +24 ✔
Total charge on product side = 6(+3) + 2(+3) = +24 ✔
Step-by-Step Process for Balancing Redox Reactions Using the Half-Reaction Method in BASIC SOLUTION
If balancing a redox reaction in a basic solution, two extra steps are required. Let’s take the following example: MnO4-(aq) + C2O42-(aq) + CO2(g) + MnO2(s) and go through all the steps we learned previously, but at the end, we’re adding two more steps.
Step 1: Split into Oxidation and Reduction Half-Reactions
Reduction half-reaction: MnO4-(aq) → MnO2(s) (Mn is reduced from +7 to +4)
Oxidation half-reaction: C2O42-(aq) → CO2(g) (C is oxidized from +3 to +4)
Step 2: Balance All Elements Except Oxygen and Hydrogen
MnO4-(aq) → MnO2(s)
C2O42-(aq) → 2CO2(g)
Step 3: Balance O by adding H2O(l)
MnO4-(aq) → MnO2(s) + 2H2O(l)
C2O42-(aq) → 2CO2(g)
Step 4: Balance H by adding H+
MnO4-(aq) + 4H+(aq) → MnO2(s) + 2H2O(l)
C2O42-(aq) → 2CO2(g)
Step 5: Balance Charge by Adding Electrons
3e- + MnO4-(aq) + 4H+(aq) → MnO2(s) + 2H2O(l)
C2O42-(aq) → 2CO2(g) + 2e-
Step 6: Make Electron Transfer Equal
(3e- + MnO4-(aq) + 4H+(aq) → MnO2(s) + 2H2O(l)) x2
(C2O42-(aq) → 2CO2(g) + 2e-) x3
6e- + 2MnO4-(aq) + 8H+(aq) → 2MnO2(s) + 4H2O(l)
3C2O42-(aq) → 6CO2(g) + 6e-
Step 7: Add the Half-Reactions Together
6e- + 2MnO4-(aq) + 8H+(aq) → 2MnO2(s) + 4H2O(l)
3C2O42-(aq) → 6CO2(g) + 6e-
2MnO4-(aq) + 8H+(aq) + 3C2O42-(aq) → 2MnO2(s) + 4H2O(l) + 6CO2(g)
At this stage, the equation is balanced if in an acidic solution. To balance in a basic solution, two more steps are added.
Step 8: Add OH- to both sides to equal the number of H+ present
2MnO4-(aq) + 8H+(aq) + 8OH-(aq) + 3C2O42-(aq) → 2MnO2(s) + 4H2O(l) + 6CO2(g) + 8OH-(aq)
Step 9: Combine H+(aq) and OH-(aq) on the same side to form H2O(l). Cancel equal amounts of H2O(l) from both sides
2MnO4-(aq) + 8H2O(l) + 3C2O42-(aq) → 2MnO2(s) + 4H2O(l) + 6CO2(g) + 8OH-(aq)
Final balanced equation: 2MnO4-(aq) + 4H2O(l) + 3C2O42-(aq) → 2MnO2(s) + 6CO2(g) + 8OH-(aq)
Check for final number of atoms and charges:
2 Mn on each side ✔
24 O on each side ✔
8 H on each side ✔
6 C on each side ✔
Total charge on reactant side = -8 ✔
Total charge on product side = -8 ✔
—
By following all these steps in order, your students will be able to balance all sorts of redox reactions using the half-reaction method! What other chemistry topics would you like us to cover in future posts? Share them in the comment section!



