Oxidation numbers, also known as oxidation states, help determine what is being oxidized and reduced…

Introducing Organic Reactions to Your Chemistry Students (Part 1)
Organic chemistry is often considered one of the most challenging topics in high school and introductory university chemistry courses. With its complex organic structures, wide range of functional groups, and seemingly endless reaction pathways, organic chemistry can quickly become overwhelming for students.
As a chemistry teacher or tutor, breaking down these concepts into clear, digestible parts is key to helping your students build confidence and deeper understanding. One of the best ways to tackle organic chemistry is by categorizing reactions. Although there are hundreds of reactions, most of them fall into a few fundamental categories. Once students understand these core reaction types, they can start to recognize patterns, predict products, and apply their knowledge in different reaction scenarios.
In this guide, we’ll cover the four major types of organic reactions and explain how they work, with tips on how to present them clearly in your online lessons.
1) Addition Reactions
In addition reactions, two molecules combine to form one product. These reactions typically involve compounds with double or triple bonds, such as alkenes and alkynes. During the reaction, one of the multiple bonds break, allowing two new atoms or groups to attach to the carbon atoms.
Markovnikov’s Rule: When adding a protic acid (like HCl, HBr, H2O) to an asymmetric alkene or alkyne, the hydrogen atom is more likely to attach to the carbon with more hydrogen atoms attached already. (“The rich get richer,” the carbon that already has more hydrogens gets the new hydrogen.) Therefore, a major and minor product will result.
Example: The addition of propene and hydrochloric acid will result in 2-chloropropane as the major product and 1-chloropropane as the minor product.
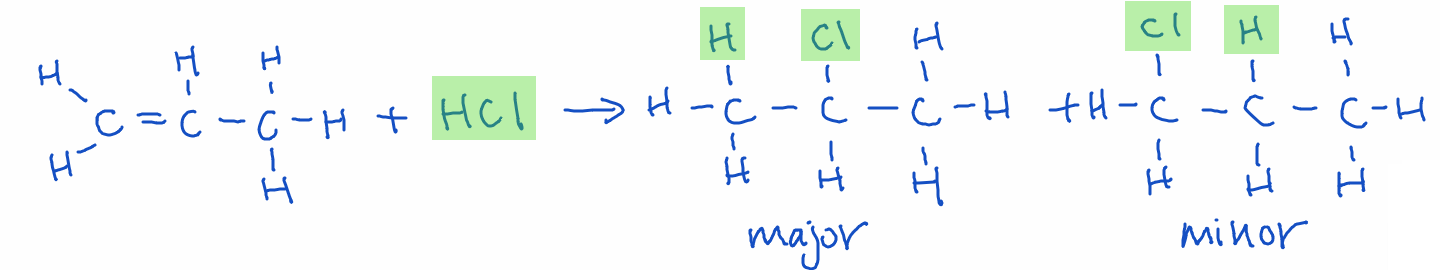
Teaching Tip: Use visual aids or reaction animations to show how the double bond breaks and new groups are added across the double bond.
2) Elimination Reactions
Elimination reactions are the opposite of addition reactions. A single molecule breaks apart to form two products, usually by removing atoms or groups from adjacent carbon atoms, resulting in the formation of a double or triple bond.
Example: Bromoethane produces ethene and hydrobromic acid.
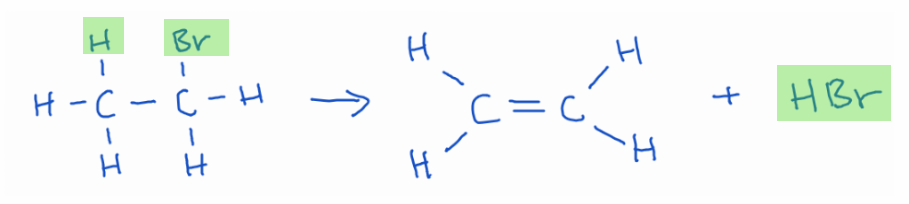
Teaching tip: Use highlighters or different color pens to show where the atoms end up.
3) Substitution Reactions
In substitution reactions, one atom or functional group in a molecule is replaced by another. These reactions are common in haloalkanes and aromatic compounds. A special type of substitution is a condensation reaction, where two organic molecules combine and release a small molecule, such as water.
Example: Esterification is when a carboxylic acid and alcohol combine to form an ester and water.
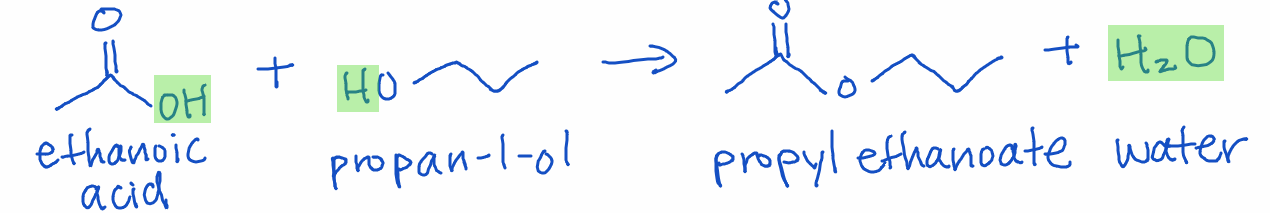
Teaching Tip: Use analogies like swapping partners at a dance to describe how the functional groups are swapped.
4) Oxidation/Reduction Reactions
These reactions involve changes in the number of bonds to oxygen or hydrogen and often occur together. In organic chemistry, oxidation typically means gaining oxygen bonds or losing hydrogen bonds, while reduction means gaining hydrogen bonds or losing oxygen bonds.
Example: The oxidation of primary alcohols to aldehydes, and further to carboxylic acids.

Teaching Tip: Since redox reactions are covered in more depth in other units, keep explanations simple and focus on recognizing changes in functional groups, such as alcohol to ketones or aldehydes.
Wrapping It All Together
Different organic compounds undergo different types of reactions depending on their functional groups and the conditions of the reaction. When teaching organic chemistry, it’s helpful to organize your lessons by both reaction type and functional group.
Try incorporating drag-and-drop activities, digital flashcards, reaction roadmaps, and whiteboard exercises during live online classes. The more you can simplify the patterns and guide students to visualize how organic molecules behave, the more confident they’ll feel when tackling more advanced organic reaction questions.
What tips and tricks do you have for introducing organic reactions to your chemistry students? Share them in the comments below!



